CPP's
News
-
Nov 30, -1
-
Feb 13, 2015
-
Feb 13, 2015
Fundamental molecular mechanism for the cellular uptake of guanidinium-rich molecules
-
Feb 13, 2015
Mechanistic Details of the Membrane Perforation and Passive Translocation of TAT Peptides
Login
Welcome Guest
- Article
- Edit
- Discussion
What are Cell-Penetrating Peptides?
Cell-Penetrating Peptides (CPPs), also known as protein transduction domains (PTDs), membrane translocating sequences (MTSs), and Trojan peptides are short peptides (≤40 amino acids), with the ability to gain access to the interior of almost any cell. They are highly cationic and usually rich in arginine and lysine amino acids. They have the exceptional property of carrying into the cells a wide variety of covalently and noncovalently conjugated cargoes such as proteins, oligonucleotides, and even 200 nm liposomes. Therefore they are extremely attractive candidates to transport drugs to the interior of cells.
What is unique about CPPs is that they are able to inject themselves into living cells crossing directly the plasma membrane. This direct mode of entry allows them and their cargoes to avoid getting trapped in endosomes. Consequently, the bioactive cargoes transported by these peptides become immediately free in the cytosol to reach their intracellular targets (immediate bioavailability).
The mechanism used by these peptides to cross into virtually any living cell in a receptor and energy independent manner was a matter of debate for many years. The core of the puzzle lied in the fact that these peptides are highly cationic and lack any hydrophobic component that would allow them to get efficiently inserted in the hydrophobic core of the plasma membrane.
The solution to this puzzle has been recently found ("Fundamental Molecular Mechanism for the Cellular Uptake of Guanidinium-Rich Molecules" published in JACS).
This work unveiled the essential role of guanidinium groups and two universal cell components: fatty acids and the cell membrane pH gradient.
It was found that the pathway employed by these peptides to get directly inserted consist in the following steps displayed in the scheme below:
a) The higher extracellular pH deprotonates fatty acids attracting arginine rich peptides.
b) The positively charged guanidinium groups bind strongly to the deprotonated carboxyl groups of fatty acids.
c) The positive charge of the peptides is now screened by the fatty acids. This allows the peptides to get efficiently inserted in the core of the plasma membrane. This insertion destabilizes the plasma membrane nucleating a transient toroidal pore.
d) The peptide diffuses on the surface of this pore towards the interior of the cell while the lower pH in the cytosol protonates the fatty acids releasing the peptide.
e) The peptide gets released and the transient pore becomes unstable and closes.
f) The protonated (and neutral) fatty acids rapidly flip-flop across the plasma membrane eventually becoming in contact with the extracellular media at higher pH where they become negatively charged and the process can start again.
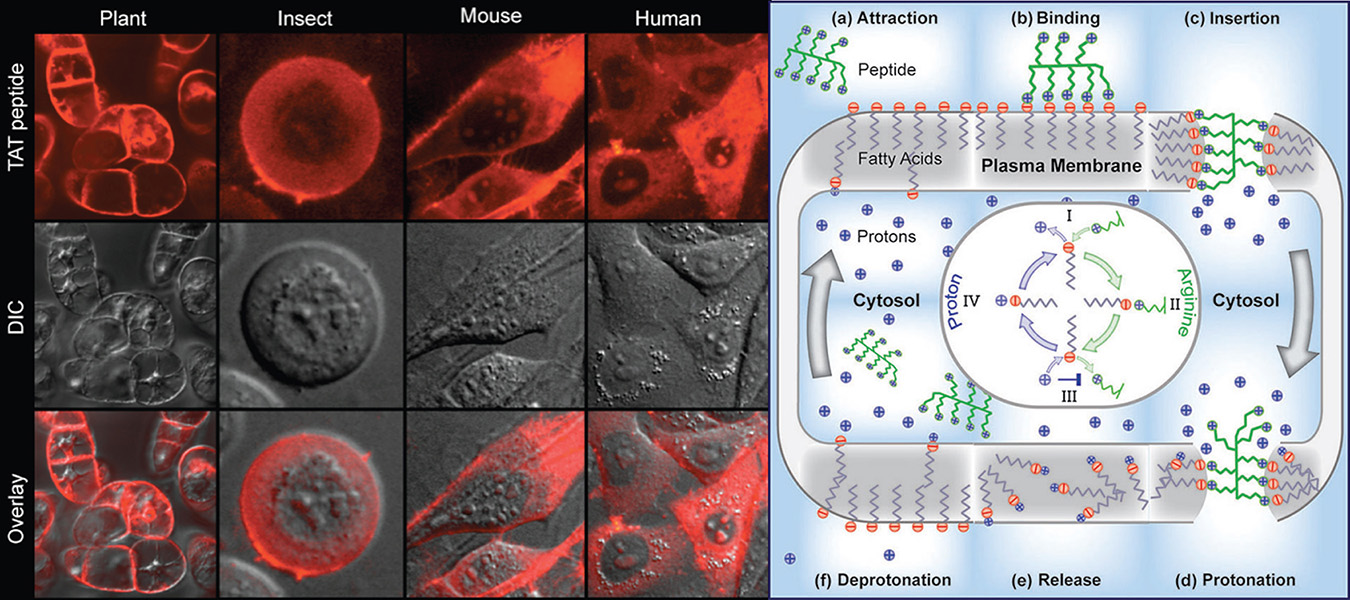
TAT peptide (labeled in red) in cells from different species and kingdoms (left panel) and scheme of the cellular uptake mechanism (right panel). (Image obtained from Herce et al. JACS, 2014)
Authors: ()
Keywords: AIDS Vaccines/*therapeutic use, Cell-Penetrating Peptides, Drug Carriers
Contact Us | Site Map | Privacy | Disclaimer | Terms & Conditions
Copyright © 2008.Cell-Penetrating Peptides | Powered by Henry David Herce